The science and chemistry of tooth decay (How and why cavities form.)
If you’re curious about the science of tooth decay formation, this page outlines what goes on. It also gives suggestions based on this information that explains steps you can take to prevent cavities from forming in your own mouth.
What causes cavities? –
1) Cavities are caused by tooth “demineralization.”
Decay formation is the result of what dentists refer to as “demineralization.” This is a process that occurs when a tooth is exposed to an acidic environment of pH 5.5 (referred to as the Critical pH) or below.
[The number 5.5 state above is an approximate value that varies with the current conditions in the person’s mouth, such as the level of calcium and phosphate in their saliva. And also the physical characteristics of their tooth enamel (its resistance to decay), which can even vary on a tooth-to-tooth basis.]
The acidic environment damages the tooth’s hard tissues (enamel and dentin) by way of leaching mineral content from them (primarily calcium and phosphate ions). Hence the term “de-mineralization.”
▲ Section references – Dean, Fejerskov
2) Where do the attacking acids come from?
The acids that cause tooth demineralization tend to be produced by specific types of bacteria, with two of the most prominent types (at least according to historic studies) being streptococci mutans and lactobacilli.
The primary home of these damaging bacteria is within dental plaque:
- S. mutans plays an instrumental role in the initiation of cavity formation due to its ability to adhere to and subsequently colonize tooth surfaces.
- As lesion formation progresses, conditions frequently shift to ones especially favorable for lactobacilli colonization. And over time, this strain often plays an ever-increasing role in the decay process.
- But with any one cavity, it’s expected that many more types of bacteria will have played a role too. With their level of presence, or even absence, fluctuating according to the current stage and specific characteristics of the ongoing lesion.
▲ Section references – Fejerskov
a) The acids that cause decay are bacterial waste products.
The bacteria that cause cavities are living organisms. And just like all living things, they consume food and in return create waste products.
As it happens, the wastes that these types of bacteria create are very acidic (having a pH of 4 and lower). The primary compound they produce, and the one primarily responsible for tooth demineralization, is lactic acid.
b) What kind of food is involved?
c) How quickly does the acid form?
The Stephan Curve.
Research by Stephan published in 1944 included a graph that illustrated the pattern of acidic response that takes place in a person’s mouth immediately after their rinsing with a sucrose (table sugar) solution.
The Stephan Curve shows what happens after you eat a sugary treat.
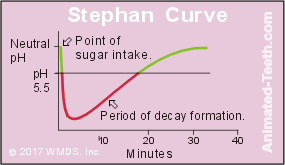
When sugar consumption causes a pH drop of 5.5 or below, decay formation can take place.
- From the moment of exposure, oral conditions immediately become increasingly acidic. A maximum value is ultimately reached in approximately 5 to 20 minutes.
- After this point, a gradual recovery, taking between 30 to 60 minutes, begins that eventually returns the mouth to its original (pre-rinse) status.
Each of the factors listed below will influence how intense the attack will be (how quickly and how much acid is produced), and therefore how long the pH of the mouth will remain below 5.5 (the level where demineralization will occur).
- The type and number of bacteria living in dental plaque. (Streptococcus mutans is the type of bacteria most associated with lactic acid formation and therefore cavity development.)
- The density of the plaque. Thicker plaque helps to protect bacteria and the acids they produce (see diagram below).
- The type of sugary meal consumed. Complex starches must be broken down into their component sugars before bacteria can digest them. In comparison, sucrose can be metabolized straight away.
▲ Section references – Stephan
Fact – Within minutes of receiving a sugary meal, oral bacteria start to produce the acids that cause tooth decay.
Cavity prevention suggestion :
The less sugar you consume, or the fewer number of times you eat sugary foods, or the less time you allow sugars to remain in your mouth, the less exposure your teeth will have to bacterial acids. Toward this goal:
- Use artificial sweeteners rather than natural sugars.
- Don’t linger when snacking on or sipping sugary foods and beverages. Consume these items fairly quickly and be done with them.
- Minimize how long sugars are allowed to remain in your mouth. Brush and floss, or at least rinse, promptly after consuming foods.
- Keep the level of plaque in your mouth to a minimum. The more cavity-causing bacteria that are present, the greater the amount of lactic acid that’s produced.
3) Factors about dental plaque that affect cavity formation.
Everyone’s mouth is inhabited by bacteria. In fact, a single human mouth can contain more microorganisms than there are people on planet Earth.
And while you can’t sterilize your mouth, there are things you can do to minimize the impact of bacteria. You do this by preventing them from forming organized colonies.
These bacterial colonies are referred to as “dental plaque,” and keeping it from building up and staying put is what brushing and flossing are all about.
a) Why plaque formation is so problematic.
Dental plaque not only provides a home for bacteria but it also acts as a medium that holds the acid they produce directly against a tooth’s surface.
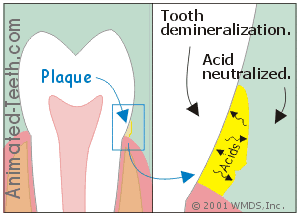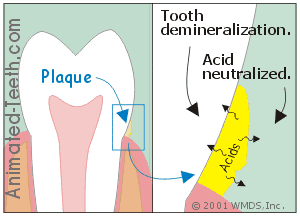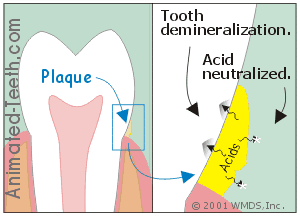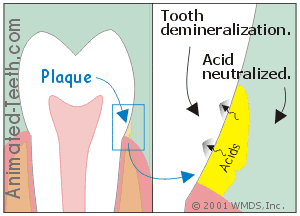
It’s the acids that leach out underneath dental plaque that cause cavities.
- It can either accumulate underneath the plaque (directly against the tooth’s surface).
- Or else seep out into the mouth.
b) Some of the acids get neutralized.
Any of the acidic waste products that seep out into the mouth won’t be able to cause decay. That’s because they’ll immediately get diluted, buffered, and/or washed away by saliva or any foods and beverages that are consumed.
c) It’s the acid underneath the plaque that causes cavities.
The acid that’s most instrumental in causing demineralization is that which seeps through the plaque and on down to the tooth’s surface.
The thickness of the dental plaque above tends to act as a protective covering that helps to shield the acid from dilution, buffering, or being washed away. Over time, saliva will finally penetrate through the dental plaque and begin to create its neutralizing effect. But this can take as long as two hours or more.
That means the acid that finds its way to the tooth’s surface will remain relatively concentrated for an extended period. During this time, demineralization will take place.
Fact – Decay occurs in those areas where dental plaque accumulates on a tooth’s surface.
Cavity prevention suggestions :
- Brush and floss often and effectively.
Take the time to be thorough with your brushing and flossing. Those places that you don’t clean well are precisely the locations where cavities will be most likely to form.
- Brush after every meal or snack. Floss at least once a day.
Fact – 1) Acid formation, and therefore tooth demineralization, begins within minutes of oral bacteria receiving a sugary meal. 2) It can take up to several hours for saliva to penetrate through dental plaque and neutralize these acids.
Cavity prevention suggestion :
Brush and floss promptly after eating so to ensure that dental plaque is not present on your teeth.
4) “Young” dental plaque is less harmful than “old.”
The amount of tooth damage that occurs after a sugar exposure is in part related to the age of the dental plaque. (Age = How long a particular glob of plaque has been sitting on a particular location on a tooth.)
Factors such as thickness, chemical nature, and the types of bacteria and their organization inside the biofilm (plaque), all correlate with its age and ability to promote the decay process.
- Studies suggest that dental biofilm must be 2 days old before it’s acid-response to an exposure to sucrose (sugar) is sufficient to cause demineralization.
(But don’t think that that means you only have to brush every two days. No human is really capable of brushing and flossing their teeth perfectly every time. So some amount of mature plaque can always be expected to lie in some nook or cranny.)
- As plaque matures, it’s characteristics change. And this can tend to inhibit otherwise effective preventive measures.
One study determined that short-term fluoride exposures (like that received from toothpaste during tooth brushing) were unable to penetrate into 7-day-old dental biofilm, thus reducing its effectiveness in preventing cavities.
▲ Section references – Fejerskov
Fact – The longer dental plaque has been sitting on a tooth’s surface, the more capable it is of causing tooth damage.
Cavity prevention suggestion :
Brush and floss often and effectively. Take the time to be thorough.
There’s more to this story …
You might be surprised to learn that the process of tooth demineralization is only half of the story about how cavities form.
It does explain how and why tooth decay starts and advances. But the whole process of cavity development is more complicated than just that. We explain on our page “How long does it take for a cavity to form?”
Tooth anatomy as it relates to cavity formation.
Every aspect of the outer surface of a tooth is composed of either enamel, dentin or cementum. They are each calcified tissues (they have a high mineral content) and are the ones in which decay forms.
But beyond that, they are very different. Here are information and some interesting facts about them.
a) Tooth Enamel
The primary hard (calcified) tissues of a tooth.
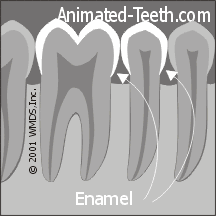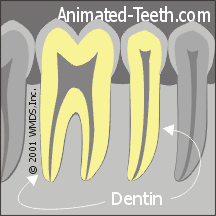
Dentin makes up the bulk of a tooth. Enamel just covers the surface of the portion lying above the gum line.
Enamel rods.
Collectively the crystals are organized into minute enamel “rods” (a tooth will have 5 to 12 million of them). These long, strong rods make up the structure of the enamel itself.
Factors affecting decay formation.
Enamel has some unique characteristics that influence how it is affected by the decay process.
- As glossy and smooth as the surface of dental enamel seems, at a microscopic level it contains pores (minute spaces that exist between its rods of hydroxyapatite).
This porosity can create differences in the density and hardness of enamel in different areas of a tooth, thus creating some locations that are more susceptible to damage by the demineralization process.
- Unlike most body tissues, after the formation of tooth enamel has been completed it’s incapable of regenerating or healing itself. That means that once an outright cavity has developed (like the case where a hole has formed) the damage is permanent.
▲ Section references – Fejerskov
b) Dentin
You might be surprised to learn that teeth are not solid enamel. It only makes up the surface layer of a tooth’s “crown” (that portion that lies at and above the gum line).
The bulk of a tooth, both its root and interior aspects, is composed of a light yellow, hard tissue called dentin (see diagram above).
It too contains a high percentage of minerals (in the form of hydroxyapatite), but not as high as enamel. Only about 70% of its composition is mineral, so relatively speaking, dentin is the “softer,” more cavity-prone, type of calcified tooth tissue.
Factors affecting decay formation.
- Dentin is damaged by a less acidic environment. Enamel undergoes demineralization at a pH 5.5 or lower. For dentin, this value is just 6.2 (or lower). (As discussed above, these values are approximate numbers that will vary according to current conditions in the person’s mouth, and physical characteristics of their teeth.)
- A part of the comparative “softness” of dentin vs. enamel has to do with the fact that its hydroxyapatite crystals are substantially smaller (10 to 30 times, in all dimensions).
- Unlike tooth enamel, dentin is a living tissue. And as such it can undergo changes in response to the decay process.
The loss of tooth structure caused by cavity formation can’t be replaced. But changes within the dentin itself or the formation of additional amounts of it within the tooth can help to create protection from advancing decay.
▲ Section references – Fejerskov
c) Cementum
Cementum is an ultra-thin mineralized layer that covers the surface of a tooth’s root.
It’s affected by the decay process, just like the other calcified tissues. But it’s so thin and destroyed so readily that we’ve confined our discussion to just dentin and enamel.
Is a tooth’s nerve involved with decay formation?
It’s only the tissues mentioned above that are directly involved with the process of tooth decay formation (a condition caused by tooth demineralization).
Yes, a tooth’s nerve (an interior, non-calcified tissue) can be damaged during the process. But that’s a secondary issue (a side effect) and fortunately doesn’t always occur.
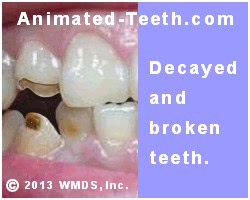
Extensive tooth decay.
Terminology – Dental caries.
“Caries” is derived from the Latin word for “rot,” which is also a suitable description of the decay process. “Dental caries” is the term you will find most frequently used in scientific literature.
FYI: Beverages you may be consuming that have a low pH.
When it comes to causing tooth damage, there’s nothing unique about the acids that bacteria create. Anything that promotes acidic conditions in your mouth assists the same demineralization process, and that includes many of the beverages that people routinely consume.
- Seventy waters and sports drinks had an average pH of 3.31.
- Fifty-one juices evaluated had an average pH of 3.48.
- Seventy-eight fruit drinks had an average pH 2.99.
- Ninety-four sodas measured had an average pH of 3.12.
- Sixty-eight energy drinks had an average pH of 3.13.
- Seventeen teas had an average pH of 3.48.
- Coffee had a pH of 5.11.
Page references sources:
Dean JA, et al. McDonald and Avery’s Dentistry for the Child and Adolescent.
Fejerskov O, et al. Dental Caries The Disease and Its Clinical Management. Chapter: Biofilms in caries development.
Pitts NB, et al. Dental caries.
Reddy A, et al. The pH of beverages in the United States.
Stephan R. Intra-oral hydrogen-ion concentrations associated with dental caries activity.
All reference sources for topic Tooth Decay.